Cart 0 Product Products (empty)
No products
Free shipping! Shipping
$0.00 Total
Product successfully added to your shopping cart
Quantity
Unit
Total
There are 0 items in your cart. There is 1 item in your cart.
Total products (tax excl.)
Total shipping (tax excl.) Free shipping!
Total (tax excl.)
Enzyme Inhibitors
- Protein Control Ligand
- Pathway Inhibitors
- Enzyme Inhibitors
- Kinase Inhibitors
- Protease
- Synthase
- p18
- p38
- p53
- p70
- p90
- Peptidase
- Carboxyl and Decarboxylases
- Ceramide Turnover Enzymes
- Chromatin Modifying Enzymes
- Cyclic Nucleotide Turnover Enzymes
- Glycerophospholipid Turnover Enzymes
- Hydroxylases
- Ubiquitin-Activating Enzyme
- Adenosine Deaminase
- Clathrin
- Nuclease
- p68
- Protein Inhibitors
- Transporter Inhibitors
- Cell Inhibition
- Synthase
- Receptor Tyrosine Phosphatases (RTP)
- Enzyme Inhibitors
- Function Modulators
- Activators
- G Protein-Coupled Receptor Ligands
- 5HT Receptors
- Adrenoceptor
- Angiotensin Receptor
- Cannabinoid Receptors
- CCK Receptors
- DA Receptors
- EAA Receptors
- Ghrelin Receptors
- GABA Receptors
- Histamine Receptors
- Leukotriene Receptors
- Metabotropic Glutamate Receptors
- Motilin Receptors
- Muscarinic Receptor
- Neuropeptide Receptors
- Opioid Receptors
- Orexin Receptors
- Orphan Receptors
- Prostanoid Receptors
- Proteinase-Activated Receptors
- Purinergic Receptors
- Ryanodine receptor
- Sigma Receptors
- Thrombin Receptor
- Vaniloid Receptor
- VIP and PACAP Receptors
- Neurotensin Receptors
- Urotensin Receptor
- Imidazoline receptor
- SMO Receptors
- Apelin Receptor
- β-arrestin/β2-adaptin
- KDM4
- Glucocorticoid Receptor
- Laminin Receptor
- Nuclear Receptor Ligands
- GDNF Receptors
- TNF Receptors
- Transcription Factors
- Chemokines
- Cytokine Receptors
- Biomarkers and Buffer Solutions
- Molecular Probes
- Stem Cell Research
- Alzheimer's Disease
- Apoptosis
- Cancer Research
- Epigenetics
- Metabolites
- PET/SPECT Imaging Precursors
- Customized Screening Library
- Ultra Pure Pharmacological Standard
- Tissue Microarray (TMA)
- Proteins and Antibodies
- Primary Cells
- ELISA KIT
- Natural Products
- Lab Equipments
- Humanized Mice for PDX Platform
- Rare Chemicals
- Custom Synthesis
 View larger
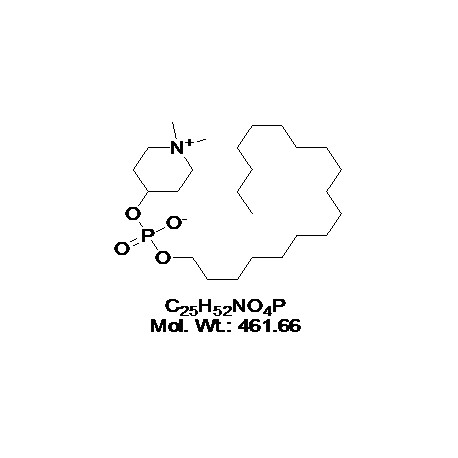
View larger Perifosine
AOB3006
CAS: 157716-52-4
Chemical Name: KRX-0401; NSC-639966; D-21266; NKA17; (1,1-Dimethylpiperidin-1-ium-4-yl) octadecyl phosphate
Molarity Calculation Cart®
HOW TO ORDER
Quantity Discount Table - Order More To Get More Price Discount
| Quantity | mg | Unit Price ($/mg or $/Unit) | Final Price |
|---|---|---|---|
| 1 | 100 | $4.95 | Total: $495.00 |
| 1 | 50 | $5.72 | Total: $286.00 |
| 1 | 25 | $6.71 | Total: $167.75 |
| 1 | 10 | $7.92 | Total: $79.20 |
| 1 | 5 | $9.35 | Total: $46.75 |
Data sheet
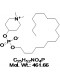
| Molecular Formula | C25H52NO4P |
| Molecular Weight | 461.66 |
| CAS Numbers | 157716-52-4 |
| Storage Condition | 0°C (short term), -20°C (long term), desiccated |
| Solubility | DMSO |
| Purity | 98% by HPLC |
| Synonym | KRX-0401; KRX 0401; KRX0401; NKA17; NSC639966; NSC 639966; NSC-639966; D 21266; D-21266; Perifosine |
| IUPAC/Chemical Name | 1,1-dimethylpiperidin-1-ium-4-yl octadecyl phosphate. |
| InChl Key | SZFPYBIJACMNJV-UHFFFAOYSA-N |
| InChl Code | InChI=1S/C25H52NO4P/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-24-29-31(27,28)30-25-20-22-26(2,3)23-21-25/h25H,4-24H2,1-3H3 |
| SMILES Code | O=P(OC1CC[N+](C)(C)CC1)([O-])OCCCCCCCCCCCCCCCCCC |
| References | 1) Amantini C, et al., Sorafenib induces cathepsin B-mediated apoptosis of bladder cancer cells by regulating the Akt/PTEN pathway. The Akt inhibitor, perifosine, enhances the sorafenib-induced cytotoxicity against bladder cancer cells. Oncoscience. 2015 Mar 23;2(4):395-409. eCollection 2015. PubMed PMID: 26097873; PubMed Central PMCID: PMC4468325.2) Kim MN, Ro SW, Kim DY, Kim DY, Cho KJ, Park JH, Lim HY, Han KH. Efficacy of perifosine alone and in combination with sorafenib in an HrasG12V plus shp53 transgenic mouse model of hepatocellular carcinoma. Cancer Chemother Pharmacol. 2015 Jun 3. [Epub ahead of print] PubMed PMID: 26037205. |
More info
Novel akt and UCHL3 inhibitor, enhancing PARP inhibitor cytotoxicity through inhibition of homologous recombination-mediated DNA double strand break repair