Cart 0 Product Products (empty)
No products
Free shipping! Shipping
$0.00 Total
Product successfully added to your shopping cart
Quantity
Unit
Total
There are 0 items in your cart. There is 1 item in your cart.
Total products (tax excl.)
Total shipping (tax excl.) Free shipping!
Total (tax excl.)
Alzheimer's Disease
- Protein Control Ligand
- Pathway Inhibitors
- Enzyme Inhibitors
- Kinase Inhibitors
- Protease
- Synthase
- p18
- p38
- p53
- p70
- p90
- Peptidase
- Carboxyl and Decarboxylases
- Ceramide Turnover Enzymes
- Chromatin Modifying Enzymes
- Cyclic Nucleotide Turnover Enzymes
- Glycerophospholipid Turnover Enzymes
- Hydroxylases
- Ubiquitin-Activating Enzyme
- Adenosine Deaminase
- Clathrin
- Nuclease
- p68
- ACE
- COX
- DHFR
- Neprilysin
- NF-κB
- RAF
- RAS
- Reductase
- ROR
- Topoisomerase
- Transferase
- Protein Inhibitors
- Transporter Inhibitors
- Cell Inhibition
- Synthase
- Receptor Tyrosine Phosphatases (RTP)
- AChE
- Peptidase
- Autophagy
- Toll-Like Receptor (TLR)
- Enzyme Inhibitors
- Function Modulators
- Activators
- G Protein-Coupled Receptor Ligands
- 5HT Receptors
- Adrenoceptor
- Angiotensin Receptor
- Cannabinoid Receptors
- CCK Receptors
- DA Receptors
- EAA Receptors
- Ghrelin Receptors
- GABA Receptors
- Histamine Receptors
- Leukotriene Receptors
- Metabotropic Glutamate Receptors
- Motilin Receptors
- Muscarinic Receptor
- Neuropeptide Receptors
- Opioid Receptors
- Orexin Receptors
- Orphan Receptors
- Prostanoid Receptors
- Proteinase-Activated Receptors
- Purinergic Receptors
- Ryanodine receptor
- Sigma Receptors
- Thrombin Receptor
- Vaniloid Receptor
- VIP and PACAP Receptors
- Neurotensin Receptors
- Urotensin Receptor
- Imidazoline receptor
- SMO Receptors
- Apelin Receptor
- β-arrestin/β2-adaptin
- KDM4
- Glucocorticoid Receptor
- Laminin Receptor
- AHR
- Amylin Receptor
- Bombesin Receptor
- Bradykinin Receptor
- CFTR
- CGRP Receptor
- CRFR
- Endothelin Receptor
- Ephrin Receptor
- Farnesoid X receptor (FXR)
- Glucagon Receptor
- Nuclear Receptor Ligands
- GDNF Receptors
- TNF Receptors
- Transcription Factors
- Chemokines
- Cytokine Receptors
- Biomarkers and Buffer Solutions
- Molecular Probes
- Stem Cell Research
- Alzheimer's Disease
- Apoptosis
- Cancer Research
- Epigenetics
- Metabolites
- PET/SPECT Imaging Precursors
- Customized Screening Library
- Ultra Pure Pharmacological Standard
- Tissue Microarray (TMA)
- Proteins and Antibodies
- Primary Cells
- ELISA KIT
- Natural Products
- Lab Equipments
- Humanized Mice for PDX Platform
- Rare Chemicals
- Custom Synthesis
- Antibacterial
- Antifungal
- Antioxidant
- Antiviral
- Molecular Glues
- PROTAC Linker
- SARS-CoV
 View larger
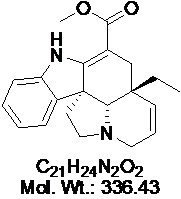
View larger Tabersonine
AOB5150
CAS: 4429-63-4
Chemical Name: Methyl (5alpha,19alpha)-16-methoxy-8-oxo-2,3,6,7-tetradehydroaspidospermidine-3-carboxylate
990 Items
Molarity Calculation Cart®
HOW TO ORDER
Quantity Discount Table - Order More To Get More Price Discount
| Quantity | mg | Unit Price ($/mg or $/Unit) | Final Price |
|---|---|---|---|
| 1 | 5 | $12.75 | Total: $63.75 |
| 1 | 10 | $10.80 | Total: $108.00 |
| 1 | 25 | $9.15 | Total: $228.75 |
| 1 | 50 | $7.80 | Total: $390.00 |
| 1 | 100 | $6.75 | Total: $675.00 |
Data sheet
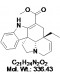
| Molecular Formula | C21H24N2O2 |
| Molecular Weight | 336.4 |
| CAS Numbers | 4429-63-4 |
| Storage Condition | 0°C (short term), -20°C (long term), desiccated |
| Solubility | DMSO |
| Purity | 98% by HPLC |
| Synonym | (–)-Tabersonine |
| IUPAC/Chemical Name | (5α,12R,19α)-2,3,6,7-tetradehydro-aspidospermidine-3-carboxylic acid, methyl ester |
| InChl Key | FNGGIPWAZSFKCN-ACRUOGEOSA-N |
| InChl Code | InChI=1S/C21H24N2O2/c1-3-20-9-6-11-23-12-10-21(19(20)23)15-7-4-5-8-16(15)22-17(21)14(13-20)18(24)25-2/h4-9,19,22H,3,10-13H2,1-2H3/t19-,20-,21-/m0/s1 |
| SMILES Code | O=C(OC)C1=C2[C@]3(C4=CC=CC=C4N2)[C@@]5([H])N(CC3)CC=C[C@@]5(CC)C1 |
| References | 1) 1. Kai, T., Zhang, L., Wang, X., et al. Tabersonine inhibits amyloid fibril formation and cytotoxicity of Aβ(1-42). ACS Chem. Neurosci. 6(6), 879-888 (2015). 2) Qu, Y., Easson, M.L.A.E., Froese, J., et al. Completion of the seven-step pathway from tabersonine to the anticancer drug precursor vindoline and its assembly in yeast. Proc. Natl. Acad. Sci. U.S.A. 112(19), 6224-6229 (2015). |
More info
Inhibitor of Amyloid Fibril Formation and Cytotoxicity of Aβ(1-42), permeating the blood-brain barrier