Cart 0 Product Products (empty)
No products
Free shipping! Shipping
$0.00 Total
Product successfully added to your shopping cart
Quantity
Unit
Total
There are 0 items in your cart. There is 1 item in your cart.
Total products (tax excl.)
Total shipping (tax excl.) Free shipping!
Total (tax excl.)
G Protein-Coupled Receptor Ligands
- Protein Control Ligand
- Pathway Inhibitors
- Enzyme Inhibitors
- Kinase Inhibitors
- Protease
- Synthase
- p18
- p38
- p53
- p70
- p90
- Peptidase
- Carboxyl and Decarboxylases
- Ceramide Turnover Enzymes
- Chromatin Modifying Enzymes
- Cyclic Nucleotide Turnover Enzymes
- Glycerophospholipid Turnover Enzymes
- Hydroxylases
- Ubiquitin-Activating Enzyme
- Adenosine Deaminase
- Clathrin
- Nuclease
- p68
- ACE
- COX
- DHFR
- Neprilysin
- NF-κB
- RAF
- RAS
- Reductase
- ROR
- Topoisomerase
- Transferase
- Protein Inhibitors
- Transporter Inhibitors
- Cell Inhibition
- Synthase
- Receptor Tyrosine Phosphatases (RTP)
- AChE
- Peptidase
- Autophagy
- Toll-Like Receptor (TLR)
- Enzyme Inhibitors
- Function Modulators
- Activators
- G Protein-Coupled Receptor Ligands
- 5HT Receptors
- Adrenoceptor
- Angiotensin Receptor
- Cannabinoid Receptors
- CCK Receptors
- DA Receptors
- EAA Receptors
- Ghrelin Receptors
- GABA Receptors
- Histamine Receptors
- Leukotriene Receptors
- Metabotropic Glutamate Receptors
- Motilin Receptors
- Muscarinic Receptor
- Neuropeptide Receptors
- Opioid Receptors
- Orexin Receptors
- Orphan Receptors
- Prostanoid Receptors
- Proteinase-Activated Receptors
- Purinergic Receptors
- Ryanodine receptor
- Sigma Receptors
- Thrombin Receptor
- Vaniloid Receptor
- VIP and PACAP Receptors
- Neurotensin Receptors
- Urotensin Receptor
- Imidazoline receptor
- SMO Receptors
- Apelin Receptor
- β-arrestin/β2-adaptin
- KDM4
- Glucocorticoid Receptor
- Laminin Receptor
- AHR
- Amylin Receptor
- Bombesin Receptor
- Bradykinin Receptor
- CFTR
- CGRP Receptor
- CRFR
- Endothelin Receptor
- Ephrin Receptor
- Farnesoid X receptor (FXR)
- Glucagon Receptor
- Nuclear Receptor Ligands
- GDNF Receptors
- TNF Receptors
- Transcription Factors
- Chemokines
- Cytokine Receptors
- Biomarkers and Buffer Solutions
- Molecular Probes
- Stem Cell Research
- Alzheimer's Disease
- Apoptosis
- Cancer Research
- Epigenetics
- Metabolites
- PET/SPECT Imaging Precursors
- Customized Screening Library
- Ultra Pure Pharmacological Standard
- Tissue Microarray (TMA)
- Proteins and Antibodies
- Primary Cells
- ELISA KIT
- Natural Products
- Lab Equipments
- Humanized Mice for PDX Platform
- Rare Chemicals
- Custom Synthesis
- Antibacterial
- Antifungal
- Antioxidant
- Antiviral
- Molecular Glues
- PROTAC Linker
- SARS-CoV
 View larger
View larger Quinabactin
AOB6476
CAS: 946270-26-4
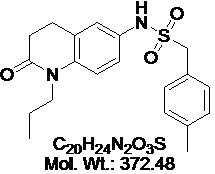
Chemical Name: LC-66C6; ABA mimics 1; AM1; N-(2-oxo-1-propyl-1,2,3,4-tetrahydroquinolin-6-yl)-1-p-tolylmethanesulfonamide
237 Items
Molarity Calculation Cart®
HOW TO ORDER
Quantity Discount Table - Order More To Get More Price Discount
| Quantity | mg | Unit Price ($/mg or $/Unit) | Final Price |
|---|---|---|---|
| 1 | 5 | $50.15 | Total: $250.75 |
| 1 | 10 | $42.48 | Total: $424.80 |
| 1 | 25 | $35.99 | Total: $899.75 |
| 1 | 50 | $30.68 | Total: $1,534.00 |
| 1 | 100 | $26.55 | Total: $2,655.00 |
Data sheet
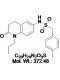
| Molecular Formula | C20H24N2O3S |
| Molecular Weight | 372.48 |
| CAS Numbers | 946270-26-4 |
| Storage Condition | 0°C (short term), -20°C (long term), desiccated |
| Solubility | DMSO |
| Purity | 98% by HPLC |
| Synonym | Quinabactin; LC-66C6; LC 66C6; LC66C6; ABA mimics 1; AM1; AM-1; AM 1; |
| IUPAC/Chemical Name | N-(2-oxo-1-propyl-1,2,3,4-tetrahydroquinolin-6-yl)-1-p-tolylmethanesulfonamide |
| InChl Key | IVHKSUMLZQXFPR-UHFFFAOYSA-N |
| InChl Code | InChI=1S/C20H24N2O3S/c1-3-12-22-19-10-9-18(13-17(19)8-11-20(22)23)21-26(24,25)14-16-6-4-15(2)5-7-16/h4-7,9-10,13,21H,3,8,11-12,14H2,1-2H3 |
| SMILES Code | O=S(CC1=CC=C(C)C=C1)(NC2=CC3=C(N(CCC)C(CC3)=O)C=C2)=O |
| References | 1) Vaidya AS, et al., A Rationally Designed Agonist Defines Subfamily IIIA Abscisic Acid Receptors As Critical Targets for Manipulating Transpiration. ACS Chem Biol. 2017 Nov 17;12(11):2842-2848.2) Helander JD, et al.,. Chemical manipulation of plant water use. Bioorg Med Chem. 2016 Feb 1;24(3):493-500. |
More info
Aba-mimicking agonist, preferentially activating dimeric ABA receptors and possesses ABA-like potency in vivo