No products
Product successfully added to your shopping cart
There are 0 items in your cart. There is 1 item in your cart.
Primary Cells
- Protein Control Ligand
- Pathway Inhibitors
- Enzyme Inhibitors
- Kinase Inhibitors
- Protease
- Synthase
- p18
- p38
- p53
- p70
- p90
- Peptidase
- Carboxyl and Decarboxylases
- Ceramide Turnover Enzymes
- Chromatin Modifying Enzymes
- Cyclic Nucleotide Turnover Enzymes
- Glycerophospholipid Turnover Enzymes
- Hydroxylases
- Ubiquitin-Activating Enzyme
- Adenosine Deaminase
- Clathrin
- Nuclease
- p68
- ACE
- COX
- DHFR
- Neprilysin
- NF-κB
- RAF
- RAS
- Reductase
- ROR
- Topoisomerase
- Transferase
- Protein Inhibitors
- Transporter Inhibitors
- Cell Inhibition
- Synthase
- Receptor Tyrosine Phosphatases (RTP)
- AChE
- Peptidase
- Autophagy
- Toll-Like Receptor (TLR)
- Enzyme Inhibitors
- Function Modulators
- Activators
- G Protein-Coupled Receptor Ligands
- 5HT Receptors
- Adrenoceptor
- Angiotensin Receptor
- Cannabinoid Receptors
- CCK Receptors
- DA Receptors
- EAA Receptors
- Ghrelin Receptors
- GABA Receptors
- Histamine Receptors
- Leukotriene Receptors
- Metabotropic Glutamate Receptors
- Motilin Receptors
- Muscarinic Receptor
- Neuropeptide Receptors
- Opioid Receptors
- Orexin Receptors
- Orphan Receptors
- Prostanoid Receptors
- Proteinase-Activated Receptors
- Purinergic Receptors
- Ryanodine receptor
- Sigma Receptors
- Thrombin Receptor
- Vaniloid Receptor
- VIP and PACAP Receptors
- Neurotensin Receptors
- Urotensin Receptor
- Imidazoline receptor
- SMO Receptors
- Apelin Receptor
- β-arrestin/β2-adaptin
- KDM4
- Glucocorticoid Receptor
- Laminin Receptor
- AHR
- Amylin Receptor
- Bombesin Receptor
- Bradykinin Receptor
- CFTR
- CGRP Receptor
- CRFR
- Endothelin Receptor
- Ephrin Receptor
- Farnesoid X receptor (FXR)
- Glucagon Receptor
- Nuclear Receptor Ligands
- GDNF Receptors
- TNF Receptors
- Transcription Factors
- Chemokines
- Cytokine Receptors
- Biomarkers and Buffer Solutions
- Molecular Probes
- Stem Cell Research
- Alzheimer's Disease
- Apoptosis
- Cancer Research
- Epigenetics
- Metabolites
- PET/SPECT Imaging Precursors
- Customized Screening Library
- Ultra Pure Pharmacological Standard
- Tissue Microarray (TMA)
- Proteins and Antibodies
- Primary Cells
- ELISA KIT
- Natural Products
- Lab Equipments
- Humanized Mice for PDX Platform
- Rare Chemicals
- Custom Synthesis
- Antibacterial
- Antifungal
- Antioxidant
- Antiviral
- Molecular Glues
- PROTAC Linker
- SARS-CoV
 View larger
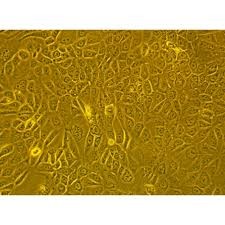
View larger Human Primary Esophageal Fibroblasts Cells
HUM-d029
Each vial contains >5x105 cells in 1mL volume
1000 Items
Molarity Calculation Cart®
HOW TO ORDER
More info
Cell Details
Esophageal fibroma originates from the esophageal muscular layer, most of which is located in the lower esophagus. The tumor is hard and expands. The general course of disease is long, ranging from several months to several years. It is the most common benign esophageal tumor, multiple. Isolation and culture of esophageal fibroma cells in vitro laid the experimental foundation for studying the biological characteristics and mechanism of esophageal fibroids.
Cell Characteristics
1) The cells are derived from human esophageal tumor tissue.
2) Does not contain HIV-1, HBV, HCV, mycoplasma, bacteria, yeast and fungi.
3) Cell growth mode: long fusiform, irregular cells, adherent culture.
Transportation and Preservation
Depending on the weather conditions and the distance of transportation, the company negotiates with the customer and chooses one of the following methods.
1) 1mL of frozen cell suspension is placed in a 1.8mL cryotube and placed in a foam incubator filled with dry ice for transport; after receiving the cells, thaw the resuscitated cells as soon as possible for culture. If resuscitation is not possible immediately, Cryopreserved cells can be stored at -80°C for 1 month.
2) T-25 culture flask is filled with complete medium and then transported at room temperature. After receiving the cells, please observe the growth state of the cells under a microscope. If the bottle filling rate exceeds 85%, please carry out the subculture immediately. If there are more cells in suspension, allow the flask to stand overnight in the incubator to help the undead suspension cells to reattach.
Product Use
1) This product can only be used for scientific research
2) This product has not passed the audit for living animals and humans directly.
3) This product has not passed the audit for in vivo diagnosis.

