Cart 0 Product Products (empty)
No products
Free shipping! Shipping
$0.00 Total
Product successfully added to your shopping cart
Quantity
Unit
Total
There are 0 items in your cart. There is 1 item in your cart.
Total products (tax excl.)
Total shipping (tax excl.) Free shipping!
Total (tax excl.)
Products
- Protein Control Ligand
- Pathway Inhibitors
- Enzyme Inhibitors
- Kinase Inhibitors
- Protease
- Synthase
- p18
- p38
- p53
- p70
- p90
- Peptidase
- Carboxyl and Decarboxylases
- Ceramide Turnover Enzymes
- Chromatin Modifying Enzymes
- Cyclic Nucleotide Turnover Enzymes
- Glycerophospholipid Turnover Enzymes
- Hydroxylases
- Ubiquitin-Activating Enzyme
- Adenosine Deaminase
- Clathrin
- Nuclease
- p68
- ACE
- COX
- DHFR
- Neprilysin
- NF-κB
- RAF
- RAS
- Reductase
- ROR
- Topoisomerase
- Transferase
- Protein Inhibitors
- Transporter Inhibitors
- Cell Inhibition
- Synthase
- Receptor Tyrosine Phosphatases (RTP)
- AChE
- Peptidase
- Autophagy
- Toll-Like Receptor (TLR)
- Enzyme Inhibitors
- Function Modulators
- Activators
- G Protein-Coupled Receptor Ligands
- 5HT Receptors
- Adrenoceptor
- Angiotensin Receptor
- Cannabinoid Receptors
- CCK Receptors
- DA Receptors
- EAA Receptors
- Ghrelin Receptors
- GABA Receptors
- Histamine Receptors
- Leukotriene Receptors
- Metabotropic Glutamate Receptors
- Motilin Receptors
- Muscarinic Receptor
- Neuropeptide Receptors
- Opioid Receptors
- Orexin Receptors
- Orphan Receptors
- Prostanoid Receptors
- Proteinase-Activated Receptors
- Purinergic Receptors
- Ryanodine receptor
- Sigma Receptors
- Thrombin Receptor
- Vaniloid Receptor
- VIP and PACAP Receptors
- Neurotensin Receptors
- Urotensin Receptor
- Imidazoline receptor
- SMO Receptors
- Apelin Receptor
- β-arrestin/β2-adaptin
- KDM4
- Glucocorticoid Receptor
- Laminin Receptor
- AHR
- Amylin Receptor
- Bombesin Receptor
- Bradykinin Receptor
- CFTR
- CGRP Receptor
- CRFR
- Endothelin Receptor
- Ephrin Receptor
- Farnesoid X receptor (FXR)
- Glucagon Receptor
- Nuclear Receptor Ligands
- GDNF Receptors
- TNF Receptors
- Transcription Factors
- Chemokines
- Cytokine Receptors
- Biomarkers and Buffer Solutions
- Molecular Probes
- Stem Cell Research
- Alzheimer's Disease
- Apoptosis
- Cancer Research
- Epigenetics
- Metabolites
- PET/SPECT Imaging Precursors
- Customized Screening Library
- Ultra Pure Pharmacological Standard
- Tissue Microarray (TMA)
- Proteins and Antibodies
- Primary Cells
- ELISA KIT
- Natural Products
- Lab Equipments
- Humanized Mice for PDX Platform
- Rare Chemicals
- Custom Synthesis
- Antibacterial
- Antifungal
- Antioxidant
- Antiviral
- Molecular Glues
- PROTAC Linker
- SARS-CoV
 View larger
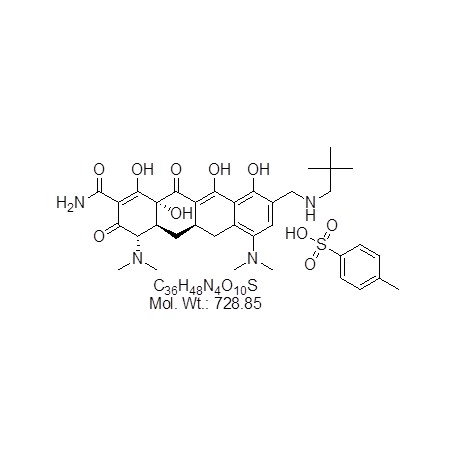
View larger Omadacycline Tosylate
AOB8700
CAS: 1075240-43-5
Chemical Name: PTK-0796; (4S,4aS,5aR,12aR)-4,7-Bis(dimethylamino)-9-[(2,2-dimethylpropylamino)methyl]-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4H-tetracene-2-carboxamide;4-methylbenzenesulfonic acid
Molarity Calculation Cart®
HOW TO ORDER
Quantity Discount Table - Order More To Get More Price Discount
| Quantity | mg | Unit Price ($/mg or $/Unit) | Final Price |
|---|---|---|---|
| 1 | 100 | $58.05 | Total: $5,805.00 |
| 1 | 50 | $67.08 | Total: $3,354.00 |
| 1 | 25 | $78.69 | Total: $1,967.25 |
| 1 | 10 | $92.88 | Total: $928.80 |
| 1 | 5 | $109.65 | Total: $548.25 |
Data sheet
| Molecular Formula | C36H48N4O10S |
| Molecular Weight | 728.85 |
| CAS Numbers | 1075240-43-5 |
| Storage Condition | 0°C (short term), -20°C (long term), desiccated |
| Solubility | DMSO and Alcohol |
| Purity | 98% by HPLC |
| Synonym | PTK 0796; PTK-0796; PTK0796; Amadacyclin; Omadacycline; Nuzyra. |
| IUPAC/Chemical Name | (4S,4aS,5aR,12aR)-4,7-Bis(dimethylamino)-9-[(2,2-dimethylpropylamino)methyl]-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4H-tetracene-2-carboxamide;4-methylbenzenesulfonic acid |
| InChl Key | JEECQCWWSTZDCK-IQZGDKDPSA-N |
| InChl Code | InChI=1S/C29H40N4O7/c1-28(2,3)12-31-11-14-10-17(32(4)5)15-8-13-9-16-21(33(6)7)24(36)20(27(30)39)26(38)29(16,40)25(37)18(13)23(35)19(15)22(14)34/h10,13,16,21,31,34,36-37,40H,8-9,11-12H2,1-7H3,(H2,30,39)/t13-,16-,21-,29-/m0/s1 |
| SMILES Code | O=C(C1=C(O)[C@@H](N(C)C)[C@@] |
| References | 1) Kovacs SJ, Ting L, Praestgaard J, Sunkara G, Sun H, Stein DS, Tanaka SK, Villano S. An Open-Label Study of the Impact of Hepatic Impairment on the Pharmacokinetics and Safety of Single Oral and Intravenous Doses of Omadacycline. Antimicrob Agents Chemother. 2020 Aug 24:AAC.01650-20. doi: 10.1128/AAC.01650-20. Epub ahead of print. PMID: 32839218. 2) McKenna M. The antibiotic paradox: why companies can't afford to create life- saving drugs. Nature. 2020 Aug;584(7821):338-341. doi: 10.1038/d41586-020-02418-x. PMID: 32814891. |
More info
First-in-class aminomethylcycline antibiotic with a broad spectrum of activity against Gram-positive and Gram-negative aerobes and anaerobes, and atypical bacterial pathogens, binding almost exclusively to the muscarinic-2 (M2) subtype acetylcholine receptor and in the SA node model, antagonizing the effect of a pan-muscarinic agonist (carbamylcholine) in a concentration-dependent manner