No products
Product successfully added to your shopping cart
There are 0 items in your cart. There is 1 item in your cart.
Proteins and Enzymes
- Protein Control Ligand
- Pathway Inhibitors
- Enzyme Inhibitors
- Kinase Inhibitors
- Protease
- Synthase
- p18
- p38
- p53
- p70
- p90
- Peptidase
- Carboxyl and Decarboxylases
- Ceramide Turnover Enzymes
- Chromatin Modifying Enzymes
- Cyclic Nucleotide Turnover Enzymes
- Glycerophospholipid Turnover Enzymes
- Hydroxylases
- Ubiquitin-Activating Enzyme
- Adenosine Deaminase
- Clathrin
- Nuclease
- p68
- ACE
- COX
- DHFR
- Neprilysin
- NF-κB
- RAF
- RAS
- Reductase
- ROR
- Topoisomerase
- Transferase
- Protein Inhibitors
- Transporter Inhibitors
- Cell Inhibition
- Synthase
- Receptor Tyrosine Phosphatases (RTP)
- AChE
- Peptidase
- Autophagy
- Toll-Like Receptor (TLR)
- Enzyme Inhibitors
- Function Modulators
- Activators
- G Protein-Coupled Receptor Ligands
- 5HT Receptors
- Adrenoceptor
- Angiotensin Receptor
- Cannabinoid Receptors
- CCK Receptors
- DA Receptors
- EAA Receptors
- Ghrelin Receptors
- GABA Receptors
- Histamine Receptors
- Leukotriene Receptors
- Metabotropic Glutamate Receptors
- Motilin Receptors
- Muscarinic Receptor
- Neuropeptide Receptors
- Opioid Receptors
- Orexin Receptors
- Orphan Receptors
- Prostanoid Receptors
- Proteinase-Activated Receptors
- Purinergic Receptors
- Ryanodine receptor
- Sigma Receptors
- Thrombin Receptor
- Vaniloid Receptor
- VIP and PACAP Receptors
- Neurotensin Receptors
- Urotensin Receptor
- Imidazoline receptor
- SMO Receptors
- Apelin Receptor
- β-arrestin/β2-adaptin
- KDM4
- Glucocorticoid Receptor
- Laminin Receptor
- AHR
- Amylin Receptor
- Bombesin Receptor
- Bradykinin Receptor
- CFTR
- CGRP Receptor
- CRFR
- Endothelin Receptor
- Ephrin Receptor
- Farnesoid X receptor (FXR)
- Glucagon Receptor
- Nuclear Receptor Ligands
- GDNF Receptors
- TNF Receptors
- Transcription Factors
- Chemokines
- Cytokine Receptors
- Biomarkers and Buffer Solutions
- Molecular Probes
- Stem Cell Research
- Alzheimer's Disease
- Apoptosis
- Cancer Research
- Epigenetics
- Metabolites
- PET/SPECT Imaging Precursors
- Customized Screening Library
- Ultra Pure Pharmacological Standard
- Tissue Microarray (TMA)
- Proteins and Antibodies
- Primary Cells
- ELISA KIT
- Natural Products
- Lab Equipments
- Humanized Mice for PDX Platform
- Rare Chemicals
- Custom Synthesis
- Antibacterial
- Antifungal
- Antioxidant
- Antiviral
- Molecular Glues
- PROTAC Linker
- SARS-CoV
 View larger
View larger Human GM-CSF / CSF2 Protein
10015-HNAH
Activity: Measured in a cell proliferation assay using TF-1 human erythroleukemic cells. The ED50 for this effect is typically 0.1-0.6 ng/ml.
1000 Items
Please ask for quote for unit smaller than 1 mg
Molarity Calculation Cart®
HOW TO ORDER
Data sheet
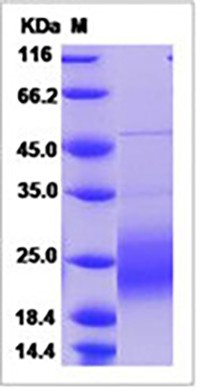
| Molecular Weight | The recombinant human GMCSF consists of 127 amino acids and predicts a molecular mass of 14.5 kDa. It migrates as an approximately 23.8 kDa band in SDS-PAGE under reducing conditions. |
| Storage Condition | Samples are stable for up to twelve months from date of receipt at -70℃. Store it under sterile conditions at -20℃ to -80℃. It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles. |
| Purity | 90% as determined by SDS-PAGE |
More info
Protein Construction: A DNA sequence encoding human GMCSF (NP_000749.2) (Met1-Glu144) was expressed.
Formulation: Lyophilized from sterile PBS, pH 7.4.1. Normally 5% - 8% trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA.2. Please contact us for any concerns or special requirements.Please refer to the specific buffer information in the hard copy of CoA.
Reconstitution: A hardcopy of COA with reconstitution instruction is sent along with the products. Please refer to it for detailed information.
GM-CSF Background Information: Granulocyte-macrophage colony-stimulating factor (GM-CSF) is one of an array of cytokines with pivotal roles in embryo implantation and subsequent development. Several cell lineages in the reproductive tract and gestational tissues synthesise GM-CSF under direction by ovarian steroid hormones and signalling agents originating in male seminal fluid and the conceptus. The pre-implantation embryo, invading placental trophoblast cells and the abundant populations of leukocytes controlling maternal immune tolerance are all subject to GM-CSF regulation. GM-CSF stimulates the differentiation of hematopoietic progenitors to monocytes and neutrophils, and reduces the risk for febrile neutropenia in cancer patients. GM-CSF also has been shown to induce the differentiation of myeloid dendritic cells (DCs) that promote the development of T-helper type 1 (cellular) immune responses in cognate T cells. The active form of the protein is found extracellularly as a homodimer, and the encoding gene is localized to a related gene cluster at chromosome region 5q31 which is known to be associated with 5q-syndrome and acute myelogenous leukemia. As a part of the immune/inflammatory cascade, GM-CSF promotes Th1 biased immune response, angiogenesis, allergic inflammation, and the development of autoimmunity, and thus worthy of consideration for therapeutic target. GM-CSF has been utilized in the clinical management of multiple disease processes. Most recently, GM-CSF has been incorporated into the treatment of malignancies as a sole therapy, as well as a vaccine adjuvant. While the benefits of GM-CSF in this arena have been promising, recent reports have suggested the potential for GM-CSF to induce immune suppression and, thus, negatively impact outcomes in the management of cancer patients. GM-CSF deficiency in pregnancy adversely impacts fetal and placental development, as well as progeny viability and growth after birth, highlighting this cytokine as a central maternal determinant of pregnancy outcome with clinical relevance in human fertility.
References:
- Robertson SA. (2007) GM-CSF regulation of embryo development and pregnancy. Cytokine Growth Factor Rev. 18(3-4): 287-98.
- Waller EK. (2007) The role of sargramostim (rhGM-CSF) as immunotherapy. Oncologist. 12 Suppl 2: 22-6.
- Clive KS, et al. (2010) Use of GM-CSF as an adjuvant with cancer vaccines: beneficial or detrimental? Expert Rev Vaccines. 9(5): 519-25.