Cart 0 Product Products (empty)
No products
Free shipping! Shipping
$0.00 Total
Product successfully added to your shopping cart
Quantity
Unit
Total
There are 0 items in your cart. There is 1 item in your cart.
Total products (tax excl.)
Total shipping (tax excl.) Free shipping!
Total (tax excl.)
Enzyme Inhibitors
- Protein Control Ligand
- Pathway Inhibitors
- Enzyme Inhibitors
- Kinase Inhibitors
- Protease
- Synthase
- p18
- p38
- p53
- p70
- p90
- Peptidase
- Carboxyl and Decarboxylases
- Ceramide Turnover Enzymes
- Chromatin Modifying Enzymes
- Cyclic Nucleotide Turnover Enzymes
- Glycerophospholipid Turnover Enzymes
- Hydroxylases
- Ubiquitin-Activating Enzyme
- Adenosine Deaminase
- Clathrin
- Nuclease
- p68
- ACE
- COX
- DHFR
- Neprilysin
- NF-κB
- RAF
- RAS
- Reductase
- ROR
- Topoisomerase
- Transferase
- Protein Inhibitors
- Transporter Inhibitors
- Cell Inhibition
- Synthase
- Receptor Tyrosine Phosphatases (RTP)
- AChE
- Peptidase
- Autophagy
- Toll-Like Receptor (TLR)
- Enzyme Inhibitors
- Function Modulators
- Activators
- G Protein-Coupled Receptor Ligands
- 5HT Receptors
- Adrenoceptor
- Angiotensin Receptor
- Cannabinoid Receptors
- CCK Receptors
- DA Receptors
- EAA Receptors
- Ghrelin Receptors
- GABA Receptors
- Histamine Receptors
- Leukotriene Receptors
- Metabotropic Glutamate Receptors
- Motilin Receptors
- Muscarinic Receptor
- Neuropeptide Receptors
- Opioid Receptors
- Orexin Receptors
- Orphan Receptors
- Prostanoid Receptors
- Proteinase-Activated Receptors
- Purinergic Receptors
- Ryanodine receptor
- Sigma Receptors
- Thrombin Receptor
- Vaniloid Receptor
- VIP and PACAP Receptors
- Neurotensin Receptors
- Urotensin Receptor
- Imidazoline receptor
- SMO Receptors
- Apelin Receptor
- β-arrestin/β2-adaptin
- KDM4
- Glucocorticoid Receptor
- Laminin Receptor
- AHR
- Amylin Receptor
- Bombesin Receptor
- Bradykinin Receptor
- CFTR
- CGRP Receptor
- CRFR
- Endothelin Receptor
- Ephrin Receptor
- Farnesoid X receptor (FXR)
- Glucagon Receptor
- Nuclear Receptor Ligands
- GDNF Receptors
- TNF Receptors
- Transcription Factors
- Chemokines
- Cytokine Receptors
- Biomarkers and Buffer Solutions
- Molecular Probes
- Stem Cell Research
- Alzheimer's Disease
- Apoptosis
- Cancer Research
- Epigenetics
- Metabolites
- PET/SPECT Imaging Precursors
- Customized Screening Library
- Ultra Pure Pharmacological Standard
- Tissue Microarray (TMA)
- Proteins and Antibodies
- Primary Cells
- ELISA KIT
- Natural Products
- Lab Equipments
- Humanized Mice for PDX Platform
- Rare Chemicals
- Custom Synthesis
- Antibacterial
- Antifungal
- Antioxidant
- Antiviral
- Molecular Glues
- PROTAC Linker
- SARS-CoV
 View larger
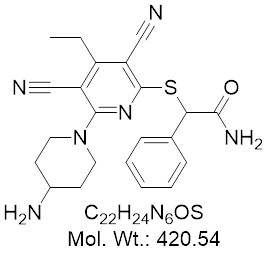
View larger GSK3685032
AOB12591
CAS: 2170137-61-6
Chemical Name: 2-((6-(4-Aminopiperidin-1-yl)-3,5-dicyano-4-ethylpyridin-2-yl)thio)-2-phenylacetamide
6859 Items
Molarity Calculation Cart®
HOW TO ORDER
Quantity Discount Table - Order More To Get More Price Discount
| Quantity | mg | Unit Price ($/mg or $/Unit) | Final Price |
|---|---|---|---|
| 1 | 5 | $17.85 | Total: $89.25 |
| 1 | 10 | $15.12 | Total: $151.20 |
| 1 | 25 | $12.81 | Total: $320.25 |
| 1 | 50 | $10.92 | Total: $546.00 |
| 1 | 100 | $9.45 | Total: $945.00 |
Data sheet
| Molecular Formula | C22H24N6OS |
| Molecular Weight | 420.54 |
| CAS Numbers | 2170137-61-6 |
| Storage Condition | 0°C (short term), -20°C (long term), desiccated |
| Purity | 99% (HPLC) |
| Synonym | GSK-3685032, GSK 3685032 |
| IUPAC/Chemical Name | 2-((6-(4-Aminopiperidin-1-yl)-3,5-dicyano-4-ethylpyridin-2-yl)thio)-2-phenylacetamide |
| References | 1) Melissa B. Pappalardi et al., Discovery of a first-in-class reversible DNMT1-selective inhibitor with improved tolerability and efficacy in acute myeloid leukemia, Nature Cancer volume 2, pages1002–1017 (2021) |
More info
First-in-class potent, non-nucleoside, reversible, DNMT1-selective inhibitor (IC50: 0.17 µM), exerting robust DNA hypomethylation and gene activation, potently inhibiting growth of cancer cell lines, displaying improved tolerability and greater DNA hypomethylation than decitabine (DAC), translating into superior antitumour activity in models of AML.